Polarized Rac-dependent protrusions drive epithelial intercalation in the embryonic epidermis of C. elegans
Elise Walck-Shannon1, David Reiner2,3, and Jeff Hardin1,4
1Graduate Program in Genetics, University of Wisconsin, 1117 West Johnson Street, Madison, Wisconsin 53706 USA 2Department of Pharmacology and Lineberger Comprehensive Cancer Center, University of North Carolina, 101 Manning Drive, Chapel Hill, NC 27514, USA 3Center for Translational Cancer Research, Institute of Biosciences and Technology and Department of Medical Physiology, Texas A&M Health Science Center, 2121 W. Holcombe Boulevard, Houston, TX 77030, USA 4Department of Zoology, University of Wisconsin, 1117 West Johnson Street, Madison, Wisconsin 53706 USA
Supplemental material for the following paper:
Walck-Shannon, E., Reiner, D., and Hardin, J. (2015). Polarized Rac-dependent protrusions drive epithelial intercalation in the embryonic epidermis of C. elegans. Development 142:3549-3560. PubMed
Summary | Movie 1 | Movie 2 | Movie 3 | Movie 4
Summary
Cell intercalation is a fundamental, coordinated cell rearrangement process that shapes tissues throughout animal development. Studies of intercalation within epithelia have focused almost exclusively on the localized constriction of specific apical junctions. Another widely deployed yet poorly understood alternative mechanism of epithelial intercalation relies on basolateral protrusive activity. Using the dorsal embryonic epidermis of Caenorhabditis elegans, we have investigated this alternative mechanism using high-resolution live cell microscopy and genetic analysis. We find that as dorsal epidermal cells migrate past one another they produce F-actin-rich protrusions polarized at their extending (medial) edges. These protrusions are controlled by the C. elegans Rac and RhoG orthologs CED-10 and MIG-2, which function redundantly to polarize actin polymerization upstream of the WAVE complex and WASP, respectively. We also identify UNC-73, the C. elegans ortholog of Trio, as a guanine nucleotide exchange factor (GEF) upstream of both CED-10 and MIG-2. Further, we identify a novel polarizing cue, CRML-1, which is the ortholog of human capping Arp2/3 myosin I linker (CARMIL), that localizes to the nonprotrusive lateral edges of dorsal cells. CRML-1 genetically suppresses UNC-73 function and, indirectly, actin polymerization. This network identifies a novel, molecularly conserved cassette that regulates epithelial intercalation via basolateral protrusive activity.
Return to top
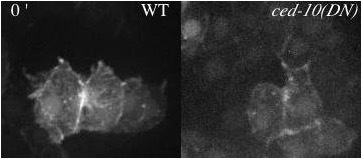
Movie 1 (295K). Protrusions in wildtype (WT) and epidermally-expressed CED-10(DN) viewed with an F-actin reporter. Minutes denoted in top left.
Download AVI | Download MP4
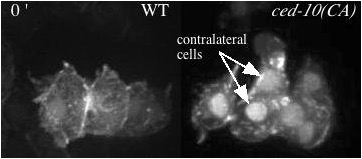
Movie 2 (208K). Protrusions in wildtype (WT) and epidermally-expressed CED-10(CA) viewed with an F-actin reporter. Minutes denoted in top left. As noted on the first frame, the ced-10(CA) embryo has an extra contralateral cell labeled with the F-actin reporter relative to the wild-type movie.
Download AVI | Download MP4
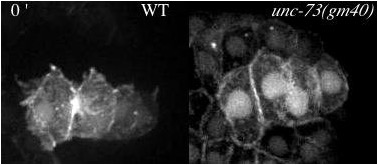
Movie 3 (190K). Protrusions in wildtype (WT) and unc-73(gm40/GEF1) viewed with an F-actin reporter. Minutes denoted in top left.
Download AVI | Download MP4
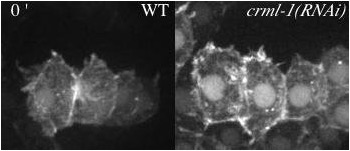
Movie 4 (136K). Protrusions in wildtype (WT) and crml-1(RNAi) viewed with an F-actin reporter. Minutes denoted in top left.
Download AVI | Download MP4
Return to top
Elise Walck-Shannon1, David Reiner2,3, and Jeff Hardin1,4
1Graduate Program in Genetics, University of Wisconsin, 1117 West Johnson Street, Madison, Wisconsin 53706 USA 2Department of Pharmacology and Lineberger Comprehensive Cancer Center, University of North Carolina, 101 Manning Drive, Chapel Hill, NC 27514, USA 3Center for Translational Cancer Research, Institute of Biosciences and Technology and Department of Medical Physiology, Texas A&M Health Science Center, 2121 W. Holcombe Boulevard, Houston, TX 77030, USA 4Department of Zoology, University of Wisconsin, 1117 West Johnson Street, Madison, Wisconsin 53706 USA
Supplemental material for the following paper:
Walck-Shannon, E., Reiner, D., and Hardin, J. (2015). Polarized Rac-dependent protrusions drive epithelial intercalation in the embryonic epidermis of C. elegans. Development 142:3549-3560. PubMed
Summary | Movie 1 | Movie 2 | Movie 3 | Movie 4
Summary
Cell intercalation is a fundamental, coordinated cell rearrangement process that shapes tissues throughout animal development. Studies of intercalation within epithelia have focused almost exclusively on the localized constriction of specific apical junctions. Another widely deployed yet poorly understood alternative mechanism of epithelial intercalation relies on basolateral protrusive activity. Using the dorsal embryonic epidermis of Caenorhabditis elegans, we have investigated this alternative mechanism using high-resolution live cell microscopy and genetic analysis. We find that as dorsal epidermal cells migrate past one another they produce F-actin-rich protrusions polarized at their extending (medial) edges. These protrusions are controlled by the C. elegans Rac and RhoG orthologs CED-10 and MIG-2, which function redundantly to polarize actin polymerization upstream of the WAVE complex and WASP, respectively. We also identify UNC-73, the C. elegans ortholog of Trio, as a guanine nucleotide exchange factor (GEF) upstream of both CED-10 and MIG-2. Further, we identify a novel polarizing cue, CRML-1, which is the ortholog of human capping Arp2/3 myosin I linker (CARMIL), that localizes to the nonprotrusive lateral edges of dorsal cells. CRML-1 genetically suppresses UNC-73 function and, indirectly, actin polymerization. This network identifies a novel, molecularly conserved cassette that regulates epithelial intercalation via basolateral protrusive activity.
Return to top

Movie 1 (295K). Protrusions in wildtype (WT) and epidermally-expressed CED-10(DN) viewed with an F-actin reporter. Minutes denoted in top left.
Download AVI | Download MP4

Movie 2 (208K). Protrusions in wildtype (WT) and epidermally-expressed CED-10(CA) viewed with an F-actin reporter. Minutes denoted in top left. As noted on the first frame, the ced-10(CA) embryo has an extra contralateral cell labeled with the F-actin reporter relative to the wild-type movie.
Download AVI | Download MP4

Movie 3 (190K). Protrusions in wildtype (WT) and unc-73(gm40/GEF1) viewed with an F-actin reporter. Minutes denoted in top left.
Download AVI | Download MP4

Movie 4 (136K). Protrusions in wildtype (WT) and crml-1(RNAi) viewed with an F-actin reporter. Minutes denoted in top left.
Download AVI | Download MP4
Return to top