
The Zinc Finger Protein DIE-1 is Required for Late Events
during Epithelial Cell Rearrangement in C. elegans
Paul J. Heid1,2, William B. Raich3,4, Ryan Smith3, William A. Mohler5,6, Steven B. Gendreau1,7, Joel H. Rothman1,8, and Jeff Hardin3,9
1 Department of Biochemistry, University of Wisconsin, Madison, WI 53706 USA
2 Present address: W.M. Keck Dynamic Image Analysis Facility, Department of Biological Sciences, University of Iowa, City, Iowa 52242
3 Program in Cellular and Molecular Biology, University of Wisconsin, Madison, WI 53706 USA
4 Present address: Center for Neurobiology and Behavior, Columbia University, 1051 Riverside Drive, New York, NY 10032
5 Laboratory of Molecular Biology, University of Wisconsin, Madison, WI 53706 USA
6 Present address: Department of Genetics and Developmental Biology, University of Connecticut, 263 Farmington Ave., Farmington, CT 06030 USA
7 Present address: Exelixis Pharmaceuticals, Inc., 170 Harbor Way, P.O. Box 511, South San Francisco, CA 94083-0511
8 Present address: Department of MCD Biology, Biological Sciences II, University of California, Santa Barbara, CA 93106 USA
9 Author for correspondence at: Department of Zoology, University of Wisconsin, 1117 W. Johnson St., Madison, WI 53706
e-mail: jdhardin@wisc.edu /voice: (608) 265-2520 /fax: (608) 262-7319
Supplemental material for the following paper:
Heid, P.J., Raich, W.B., Smith, R., Mohler, W.A., Simokat, K., Gendreau, S.B., Rothman, J.H., and Hardin, J. (2001). The zinc finger protein DIE-1 is required for late events during epithelial cell rearrangement in C. elegans. Dev Biol 236, 165-180. Medline
Summary
Results
Video sequence 1: dorsal intercalation in wild-type and die-1 embryos visualized via Nomarski microscopy
Video sequence 2: dorsal intercalation in wild-type embryos visualized via ajm-1::gfp
Video sequence 3: dorsal intercalation is defective in die-1 embryos visualized via ajm-1::gfp
Video sequence 4: defective elongation in die-1 embryos visualized via ajm-1::gfp
Video sequence 5: protrusive activity during dorsal intercalation in wild-type embryos visualized via lbp-1::gfp
Video sequence 6: cell translocation during dorsal intercalation is defective in die-1 embryos visualized via lbp-1::gfp
Summary
The mechanism by which epithelial cells undergo directed rearrangement is central to morphogenesis, yet the regulation of these movements remains poorly understood. We have investigated epithelial cell rearrangement (intercalation) in the dorsal hypodermis, or embryonic epidermis, of the C. elegans embryo by analyzing the die-1 mutant, which fails to undergo normal intercalation. Dorsal hypodermal cells of die-1(w34) homozygous embryos initiate but fail to complete the process of intercalation. Despite abnormal intercalation, the subsequent morphogenetic movements that enclose the embryo with epithelial cells and the process of dorsal cell fusion still occur. However, elongation of the embryo into a worm-like shape is disrupted in die-1 embryos, suggesting that intercalation may be necessary for subsequent elongation of the embryo. Actin filaments are not properly organized within the dorsal hypodermis of die-1 embryos, consistent with intercalation being a necessary prerequisite for elongation. The die-1 gene encodes a C2H2 zinc finger protein containing four fingers, which likely acts as a transcriptional regulator. Immunostaining with DIE-1 polyclonal antibodies and expression of a die-1::gfp translational fusion protein indicate that DIE-1 is present in the nuclei of hypodermal, muscle, and pharyngeal cells and suggest that DIE-1 acts in each of these tissues to regulate morphogenetic movements. In addition, mosaic analysis indicates that DIE-1 acts cell autonomously in the hypodermis. Our analysis of die-1 shows that the dynamic events of epithelial cell rearrangement are under transcriptional control, and shows that early and later phases of epithelial cell rearrangement are genetically distinct.
Results
Introduction to the video sequences
During the process known as dorsal intercalation, the two dorsalmost rows of embryonic epidermis, or hypodermis, of the C. elegans embryo undergo directed cell rearrangement (Williams-Masson et al., 1998). Embryos homozygous for the w34 allele of die-1 fail to complete dorsal intercalation. To analyze dorsal intercalation at the cellular level in living wild-type and die-1 mutant embryos, multiphoton excitation microscopy was used to image green fluorescent protein (GFP) fusion proteins. To analyze epithelial junctions at the cellular level, multiphoton laser scanning microscopy (MPLSM) was utilized to image a translational fusion between ajm-1(for "apical junctional molecule") and green fluorescent protein (ajm-1::gfp; AJM-1 is recognized by the monoclonal antibody MH27). The reporter was used to track epidermal cell position, junction formation, and the formation of syncytia via cell-cell fusions. Based on immunostaining and immunogold labeling, AJM-1 localizes to the apical zonula adherens, which form a belt-like structure encircling all C. elegans epithelial cells. Embryos expressing an integrated jam-1::gfp construct are phenotypically normal, and recapitulate the localization and temporal expression of endogenous AJM-1 antigen as detected by immunofluorescence with the monoclonal antibody MH27 (Mohler et al., 1998). To analyze protrusive activity during dorsal intercalation, a transcriptional fusion between the lbp-1 promoter and gfp was used (Plenefisch et al., 2000). This construct is expressed at high levels in the C-derived posterior dorsal hypodermis.
[Note: due to a nomenclature change after publication of this paper, the gene formally referred to as jam-1 was renamed ajm-1.]
Return to top
Video sequence 1:
Dorsal intercalation in wild-type and die-1 embryos visualized using Nomarski microscopy
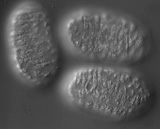
In wild-type embryos, dorsal intercalation is highly reproducible from embryo to embryo. The process can visualized using Nomarski microscopy. Cells become wedge-shaped as they begin intercalation, and eventually adopt a ladder-like arrangement straddling the dorsal midline. In contrast to wild-type embryos, intercalation is defective in homozygous die-1 (w34) embryos. Note that many of the anterior dorsal hypodermal cells intercalate, but most of the posterior dorsal cells are wedged and do not intercalate completely before they fuse into the dorsal hypodermal syncytium. A = anterior; P = posterior. Dorsal views.
Click on the link to load video sequence 1: Video sequence #1 (6 Mb)
Return to top
Video sequence 2:
Dorsal intercalation in wild-type embryos visualized using ajm-1::gfp
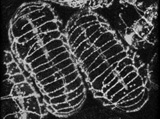
The process of dorsal intercalation can also be visualized using a translational fusion between the ajm-1 coding region (ajm = apical junctional molecule) and gfp. The embryo on the left (embryo #1) is anterior up; the embryo on the right (embryo #2) is anterior down. At the beginning of the sequence, cells in the posterior region of embryo #1 are wedging between one another. The "pointer cells" in the anterior (Williams-Masson et al., 1998) have not yet begun to wedge.
Click on the link to load video sequence 2: Video sequence #2 (9 Mb)
Return to top
Video sequence 3:
Defective dorsal intercalation in die-1 embryos visualized using ajm-1::gfp
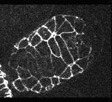
In contrast to wild-type embryos, intercalation is defective in homozygous die-1 (w34) embryos. Note that the pharynx detaches from the anterior ectoderm in this mutant as well. The anterior of the embryo is at the upper right.
Click on the link to load video sequence 3: Video sequence #3 (4.2 Mb)
Return to top
Video sequence 4:
Defective elongation in die-1 embryos visualized using ajm-1::gfp
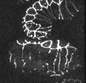
In addition to defects in dorsal intercalation, die-1 mutant embryos fail to undergo the next phases of morphogenesis, during which the embryos elongates fourfold prior to hatching into an L1 larva. Although sibling wild-type embryos elongate successfully, mutants do not. The embryo partially visible at the upper right is wildtype; the two embryos that are fully visible are die-1 mutants.
Click on the link to load video sequence 4: Video sequence #4 (5 Mb)
Return to top
Video sequence 5:
Visualizing protrusive activity in wild-type embryos during dorsal intercalation using lbp-1::gfp
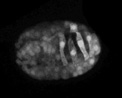
A wild-type embryo carrying the lbp-1::gfp transcriptional reporter was analyzed using multiphoton microscopy. Frames were acquired at 5 min intervals. To suppress cell fusion, the strain was made homozygous for the eff-1 (oj55) allele. Note the basolateral protrusions extended by the dorsal hypodermal cells as they intercalate. The bright ovals are the nuclei of dorsal hypodermal cells, which undergo a contralateral migration as intercalation proceeds. Anterior is to the left.
Click on the link to load video sequence 5: Video sequence #5 (1.5 Mb)
Return to top
Video sequence 6:
Cell trasnlocation is defective in die-1 mutant embryos during dorsal intercalation
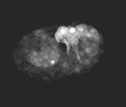
Protrusive activity in dorsal hypodermal cells in die-1 (w34) embryos was analyzed using lbp-1::gfp. Frames were acquired at 5 min intervals. To suppress cell fusion, the strain was made homozygous for the eff-1 (oj55) allele. Note that although protrusions are extended in w34 embryos, cell translocation does not occur . Anterior is to the left.
Click on the link to load video sequence 6: Video sequence #6 (3 Mb)
Return to top
References
Mohler, W. A., Simske, J. S., Williams-Masson, E. M., Hardin, J. D., and White, J. G. (1998). Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr Biol. 8, 1087-1090.
Plenefisch, J., Xiao, H., Mei, B., Geng, J., Komuniecki, P.R., and Komuniecki, R.(2000). Secretion of a novel class of iFABPs in nematodes: coordinate use of the Ascaris/Caenorhabditis model systems. Mol Biochem Parasitol. 105, 223-36.
Williams-Masson, E. M., Heid, P. J., Lavin, C. A., and Hardin, J. (1998). The cellular mechanism of epithelial rearrangement during morphogenesis of the Caenorhabditis elegans dorsal hypodermis. Dev. Biol. 204, 263-276.






