Summary
Results
Video sequence 1: AJM-1-GFP used to visualize ventral enclosure in a wild-type embryo
Video sequence 2: Loss of cadherin function specifically disrupts leading cell adhesion
Video sequence 3: HMP-1/a-catenin-GFP used to visualize nascent junction formation between leading cells in a wild-type embryo
References
Summary
Background. During embryonic development epithelia with free edges must join together to create continuous tissues that seal the interior of the organism from the outside environment; failure of epithelial sealing underlies several common human birth defects. Sealing of epithelial sheets in embryos can be extremely rapid, dramatically exceeding the rate of adherens junction formation by epithelial cells in culture or during healing of epithelial wounds. Little is known about the dynamic redistribution of cellular junctional components during such events in living embryos.
Results. We have used time-lapse, multiphoton laser scanning microscopy and a -catenin-GFP to analyze the sealing of the C. elegans epidermis in living embryos. Rapid recruitment (within 5 min) of a-catenin to sites of filopodial contact between contralateral migrating epithelial cells concomitant with clearing of cytoplasmic a-catenin results in formation of nascent junctions, and precedes the formation of mature junctions. Surprisingly, adhesion mediated by the HMR-1 cadherin and the HMP-1 a-catenin is exclusively required for adhesive strengthening between filopodia, based on analysis of hmr-1 mutants or following simultaneous inactivation of hmr-1 cadherin, hmp-1 a-catenin, and hmp-2 b-catenin. Other ventral epidermal cells, which appear to seal along the ventral midline via coordinated purse string-like contraction, successfully adhere in the absence of these proteins.
Conclusions: We propose that "filopodial priming" - prealignment of bundled actin in filopodia combined with the rapid recruitment of a-catenin from cytoplasmic reserves at sites of filopodial contact - accounts for the rapid rate of sealing of the epidermis of the C. elegans embryo. Filopodial priming may provide a general mechanism for rapid creation of adherens junctions during epithelial sheet sealing in embryos.
Return to top
Results
The cadherin/catenin complex is preferentially required in epithelial cells that interact via filopodia
Introduction to the video sequences
During the process known as ventral enclosure, the embryonic epidermis spreads from the dorsal surface of the C. elegans embryo, ultimately enclosing the embryo in an epithelial monolayer. Four cells ("leading cells") within the epidermis initiate enclosure by extending filopodial protrusions towards the ventral midline of the embryo (Williams-Masson et al., 1997). Leading cells adhere at the midline via these filopodia, followed by the coordinated contraction of more posterior ventral epidermal cells ("pocket cells") to encase the embryo in an epithelial monolayer.
To analyze ventral enclosure at the cellular level, multiphoton laser scanning microscopy (MPLSM) was utilized to image a translational fusion between AJM-1 (for "apical junctional molecule") and green fluorescent protein (AJM-1-GFP; AJM-1 is recognized by the monoclonal antibody MH27). The reporter was used to track epidermal cell position, junction formation, and the formation of syncytia via cell-cell fusions. Based on immunostaining and immunogold labeling, JAM-1 localizes to the apical zonula adherens, which form a belt-like structure encircling all C. elegans epithelial cells. Embryos expressing an integrated AJM-1-GFP construct are phenotypically normal, and recapitulate the localization and temporal expression of endogenous AJM-1 antigen as detected by immunofluorescence with the monoclonal antibody MH27 (Mohler et al., 1998).
[Note: due to a nomenclature change after publication of this paper, the gene formally referred to as jam-1 was renamed ajm-1.]
Return to top
Video sequence 1:
Ventral enclosure in wild-type embryos
In wild-type embryos, ventral enclosure is highly reproducible from embryo to embryo. Enclosure is bilaterally symmetric, with the left and right rows of ventral epidermal cells migrating at the same rate and covering the same distance. As a result, the apposing rows of epithelial cells meet in register at the ventral midline. The leading cells are the first to meet at the ventral midline, as evidenced by the formation of ventral midline junctions between each pair of cells. In the second step of ventral enclosure, 8 posterior pairs of ventral epidermal cells ("pocket cells") migrate to the ventral midline. The pocket cells complete their ventral migration ~30 minutes after the leading cells. In migrating ventral epidermal cells, AJM-1-GFP localizes to the anterior, posterior, and dorsal surfaces; the leading ventral edge does not contain detectable AJM-1-GFP.
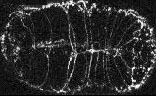
Click on the link below to load video sequence 1: Video sequence #1 (1.6 Mb)
Return to top
Video sequence 2:
Loss of cadherin function specifically disrupts leading cell adhesion
Mutations in the C. elegans cadherin/catenin complex prevent embryo elongation (Costa et al., 1998). To elucidate the mechanism of cadherin/catenin function in morphogenesis, AJM-1-GFP was observed in hmr-1, hmp-2 , or hmp-1 mutant embryos. Inactivation of hmr-1 cadherin, hmp-2 b-catenin, or hmp-1 a -catenin disrupts the adhesion of the leading cells, which are known to interact via actin-rich filopodial protrusions at the ventral midline (William-Masson et al., 1997). In contrast, the more posterior pocket cells can seal successfully at the ventral midline. An example of such an embryo, which lacks functional HMR-1 cadherin, is shown in the second video sequence.
Embryos lacking components of the cadherin/catenin complex display defects during the later stages of enclosure in the position of migrating leading cells; as the ventral epidermal cells approach the ventral midline, the pocket cells migrate in advance of the leading cells. Epidermal cells that fail to form junctions at the ventral midline retract towards the dorsal surface of the embryo. Retracting cells differ from migrating cells, as they express AJM-1-GFP at their ventral edge. Embryos deprived of both maternally provided and zygotically produced mRNA encoding one or more components of the cadherin/catenin complex maintain general cell adhesion, AJM-1-GFP localization in all epithelial tissues, and epidermal integrity. All three genes, however, are required for normal ventral enclosure, since the leading cells fail to establish successful contacts at the ventral midline in mutant or inactivated embryos. The ability of the pocket cells to establish contact further indicates that only the leading cells have a stringent requirement for cadherin-mediated adhesion.
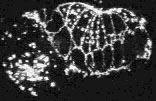
Click on the link below to load video sequence 2: Video sequence #2 (1.9 Mb)
Return to top
Video sequence 3:
HMP-1/a-catenin dynamics in wild-type embryos
In wild-type embryos, ventral enclosure initiates when four cells ("leading cells") begin migrating from a dorsolateral position toward the ventral midline. They do this by extending long filopodia, which eventually make contacty at the ventral midline. Shortly thereafter, the leading cells establish junctional connections at the midline. The earliest known marker for nascent junction formation is the accumulation of proteins of the cadherin/catenin complex. Here, we use a fully functional HMP-1/a-catenin -GFP translational fusion to image HMP-1/a-catenin accumulation at the midline in living embryos using multiphoton laser scanning microscopy (MPLSM). HMP-1/a-catenin is rapidly recruited (within 5 min) to sites of filopodial contact between contralateral migrating epithelial cells. At the same time, clearing of cytoplasmic HMP-1/a-catenin results in formation of nascent junctions, and precedes the formation of more mature junctions, characterized by the accumulation of AJM-1.
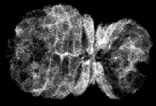
Click on the link to load video sequence 3: Video sequence #3 (1.1 Mb)
Return to top
References
Costa, M., et al. J Cell Biol 141, 297-308 (1998).
Mohler, W.A., Simske, J.S., Williams-Masson, E.M., Hardin, J.D. & White, J.G. Curr Biol 8, 1087-90 (1998).
Williams-Masson, E.M., Malik, A.N. & Hardin, J. Development 124, 2889-901 (1997).
Return to top



