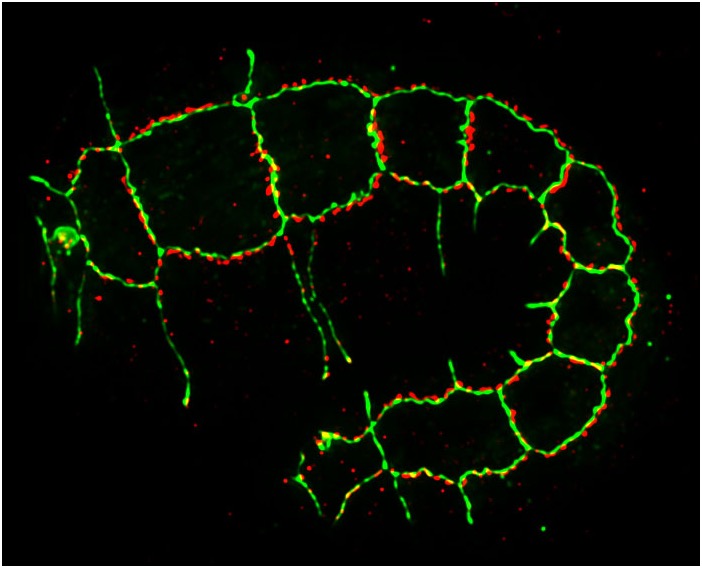
 An elongating C. elegans embryo stained for HMP-1/α-catenin (green) and AJM-1 (red) [Jeff SImske].
An elongating C. elegans embryo stained for HMP-1/α-catenin (green) and AJM-1 (red) [Jeff SImske].Morphogenesis requires that cells make strong connections with one another. We are studying how the cadherin complex, which is conserved in all metazoans, regulates morphogenesis. We are using a variety of approaches to study the cadherin complex, including:
(1) Structure-function in the embryo to identify important regions in cadherin complex proteins;
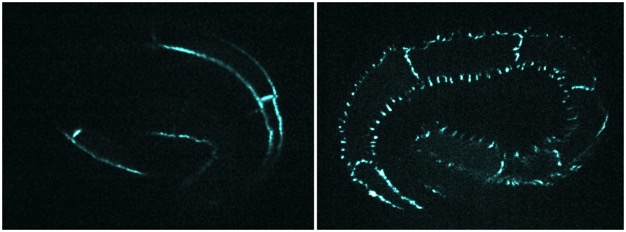 Point mutations in a conserved tyrosine in HMP-2/β-catenin to probe the importance of key regulatory residues. hmp-2(Y599F)::gfp (phospho null; left) and hmp-2(Y599E)::gfp (phosphomimetic; right) [Tim Loveless].
Point mutations in a conserved tyrosine in HMP-2/β-catenin to probe the importance of key regulatory residues. hmp-2(Y599F)::gfp (phospho null; left) and hmp-2(Y599E)::gfp (phosphomimetic; right) [Tim Loveless].(2) X-ray crystallography of the cadherin/catenin complex in collaboration with Hee-Jung Choi (Seoul National University) and BIll Weis's laboratory (Stanford University);
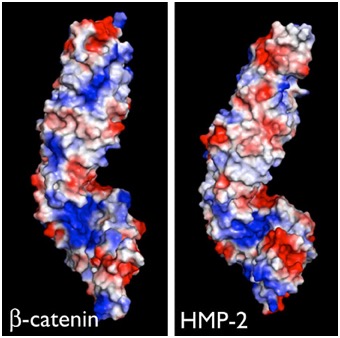 Electrostatic representation of zebrafish β-catenin (left) and C. elegans HMP-2 (right) [Hee-Jung Choi, Bill Weis, Tim Loveless].
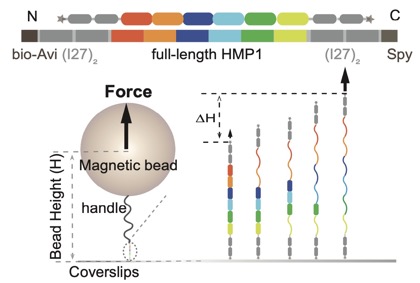
Electrostatic representation of zebrafish β-catenin (left) and C. elegans HMP-2 (right) [Hee-Jung Choi, Bill Weis, Tim Loveless].(3) Single-molecule force spectroscopy to study the mechanical properties of cadherin complex proteins in collaboration with Yan Jie's group (National University of Singapore/Mechanobioloogy Institute) to identify functionally important domains required for mechanotransduction. For an early preview of this work see this bioXriv preprint: Link.
(4) Functional genomics and proteomics to identify proteins and pathways that act in concert with the cadherin complex during morphogenesis. These include actin binding proteins and the Slit/Robo GAP, SRGP-1.
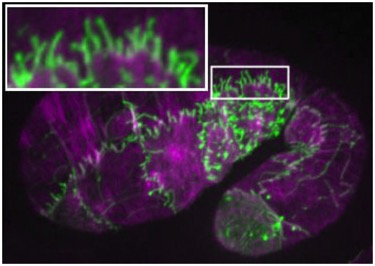 Embryo expressing SRGP-1::GFP (green) stained for actin (purple). The inset shows extensive induced tubulations. [Ronen Zaidel-Bar].
Embryo expressing SRGP-1::GFP (green) stained for actin (purple). The inset shows extensive induced tubulations. [Ronen Zaidel-Bar].
 The Hardin Lab
The Hardin Lab